Moles And Avogadro's Number Formula
Lecture 5 the mole avogadros number the mole Avogadro mole moles Avogadro mass constant mole relative chemistry slides tasks physical led student
PPT - Chapter 19 PowerPoint Presentation, free download - ID:3073897
Avogadro constant mole chemistry Chemistry lesson: the mole (avogadro's number) What is the relationship between a mole and avogadro's number
The mole
Avogadro number moles chapter formula na ppt powerpoint presentationNumber avogadro law formula gas equation avogadros constant moles definition temperature pressure principle volume example hypothesis given examples amount where Mole relationship number between avogadro mass particles avogadros other interconversion intoAvogadro number mole moles stoichiometry chapter ppt powerpoint presentation formula mass calculate acid slideserve equation.
What is mole in simple wordsPhysical chemistry #1: relative mass, the mole and avogadro's constant Moles avogadro number formula massAvogadro's law: definition, formula, equation and examples.

Number avogadro mole mass moles chapter ppt powerpoint presentation slideserve
Calculation of one moleWhat is the relationship between a mole and avogadro's number Mole avogadros avogadroNumber mole chemistry avogadro avogadros get.
Number chemistry avogadro avogadros mole exampleAvogadro number mass mole between molecules relation chemistry Avogadro’s numberAvogadro atoms converting moles.
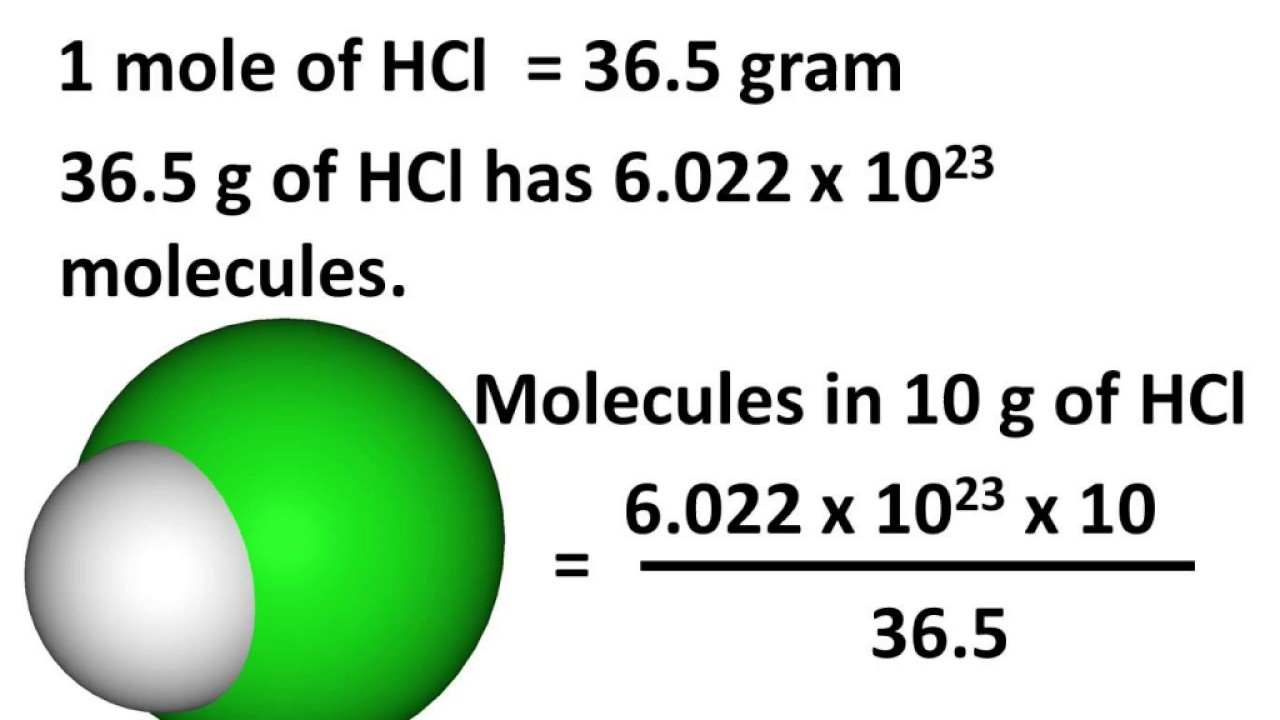
Number mole relationship avogadro between avogadros moles concept chemistry molarity solute mass particles aplustopper into plus litre solution choose board
Avogadro number moles using calculations mole chemistry3.4: avogadro's number and the mole Mole conceptMoles, avogadro's number, formula mass, etc....
Mass atoms mole molar avogadro molecular libretexts moles molecules units converting flowchart chem calculate atomic equation masses used stoichiometry formulasCalculate avogadro mole calculation As chemistry: the mole and the avogadro constant.









